- What is Cell Ag?
- Acellular Products
- Cellular Products
- The Benefits
Cellular agriculture is the production of animal-sourced foods from cell culture.
There are two kinds of agricultural products derived from cell culture: acellular products and cellular products. Acellular products are made of organic molecules like proteins and fats and contain no cellular or living material in the final product. Cellular products are made of living or once-living cells.
Products harvested from cell culture are exactly the same as those harvested from an animal (or a plant, as you’ll discover below!); the only difference is how they are made.
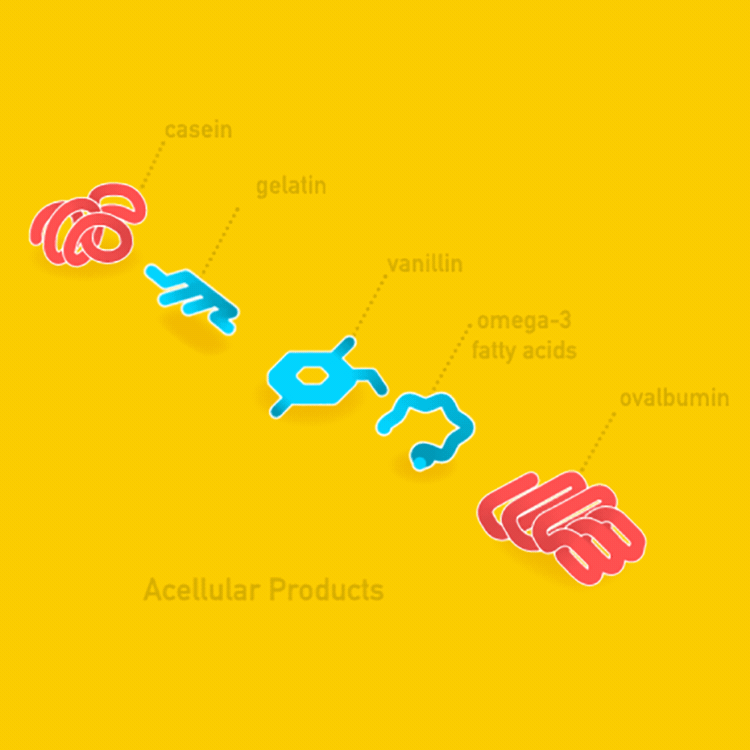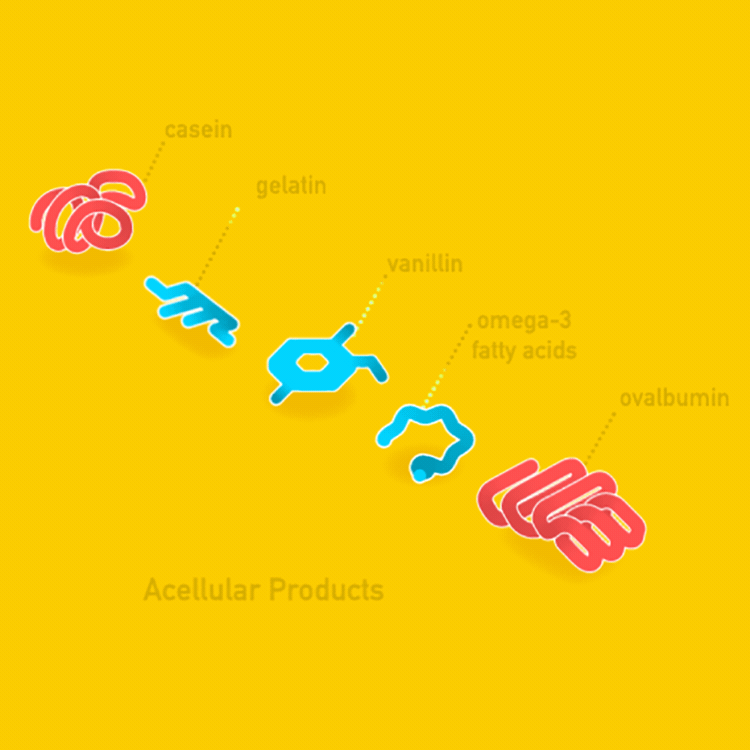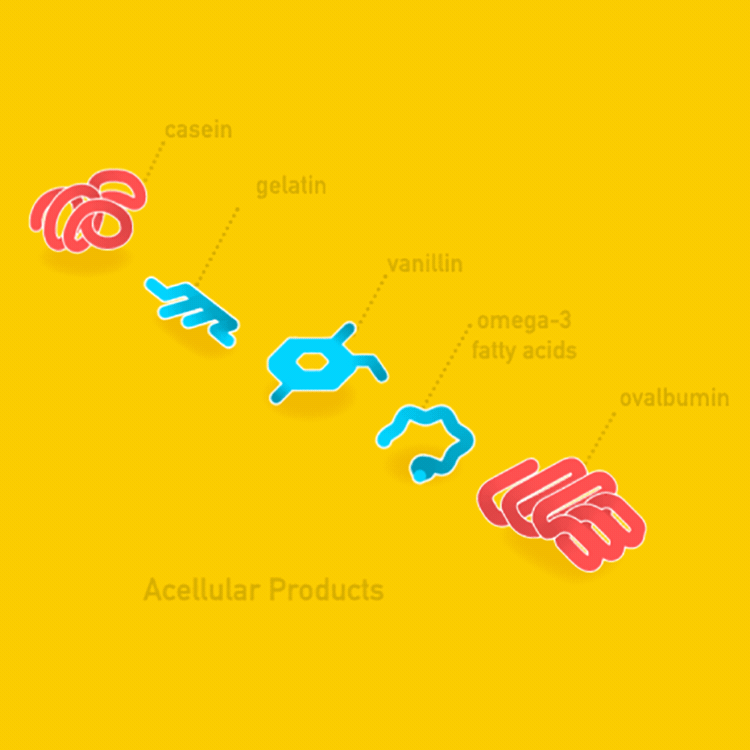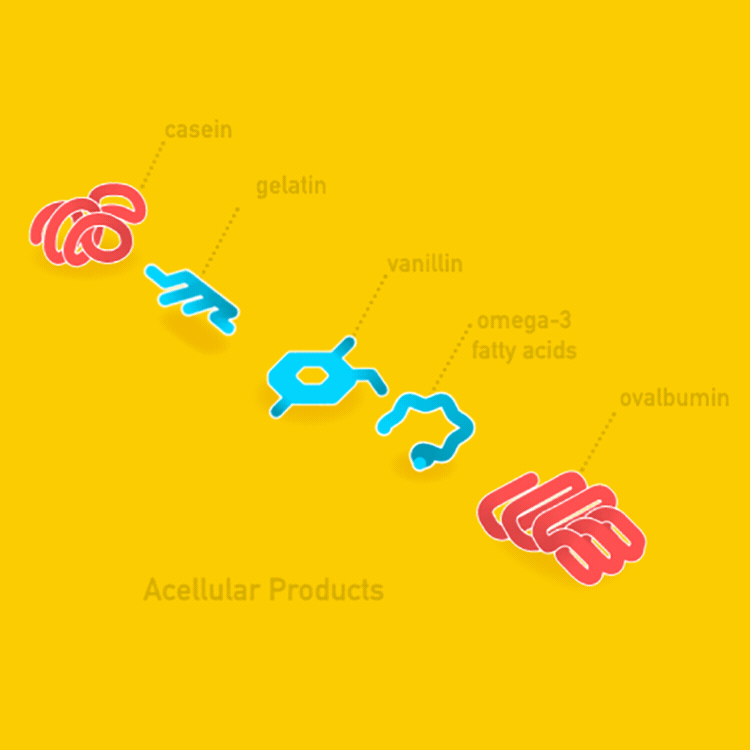
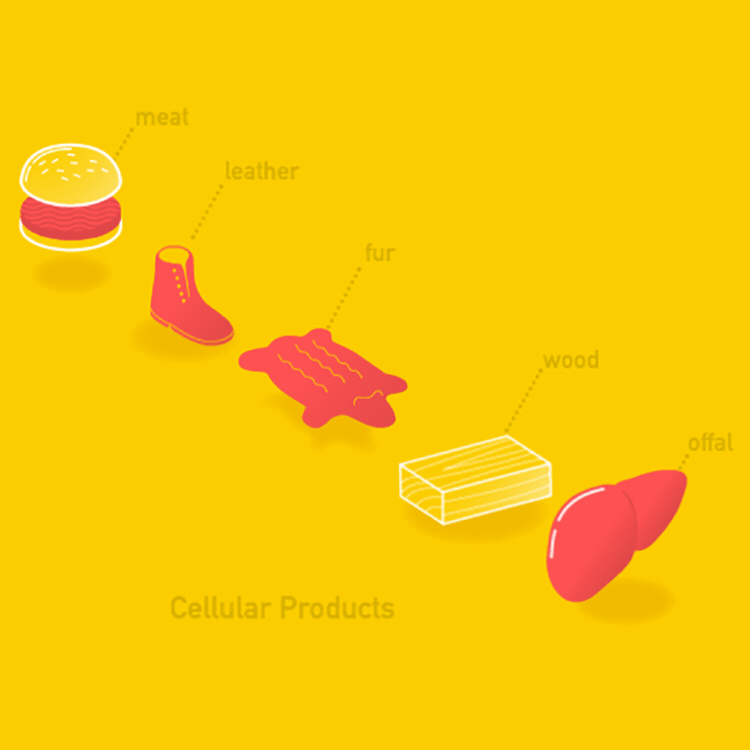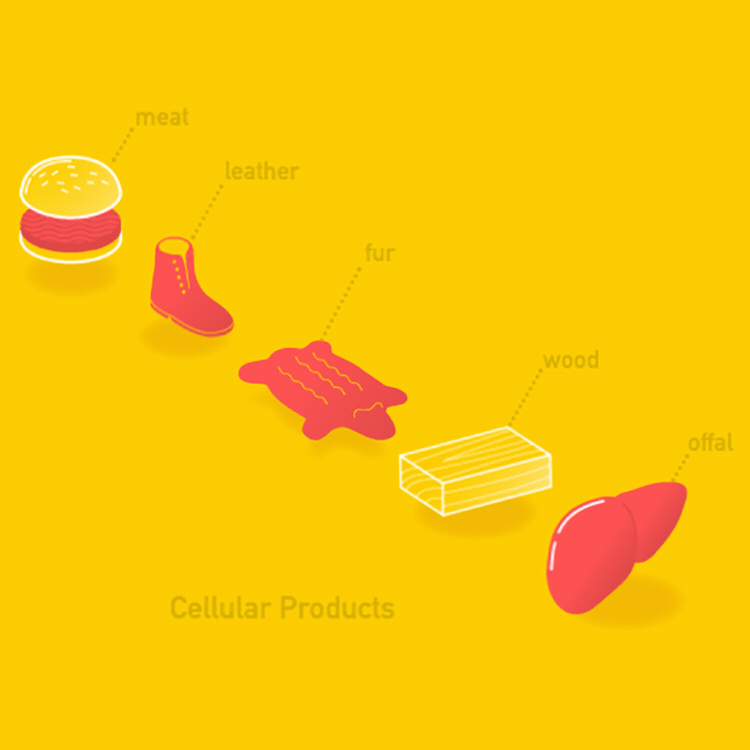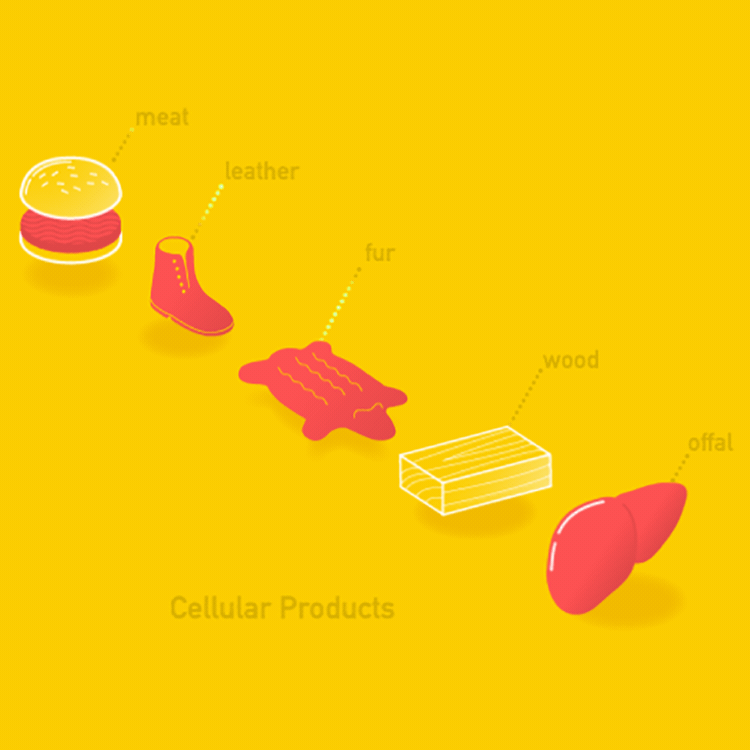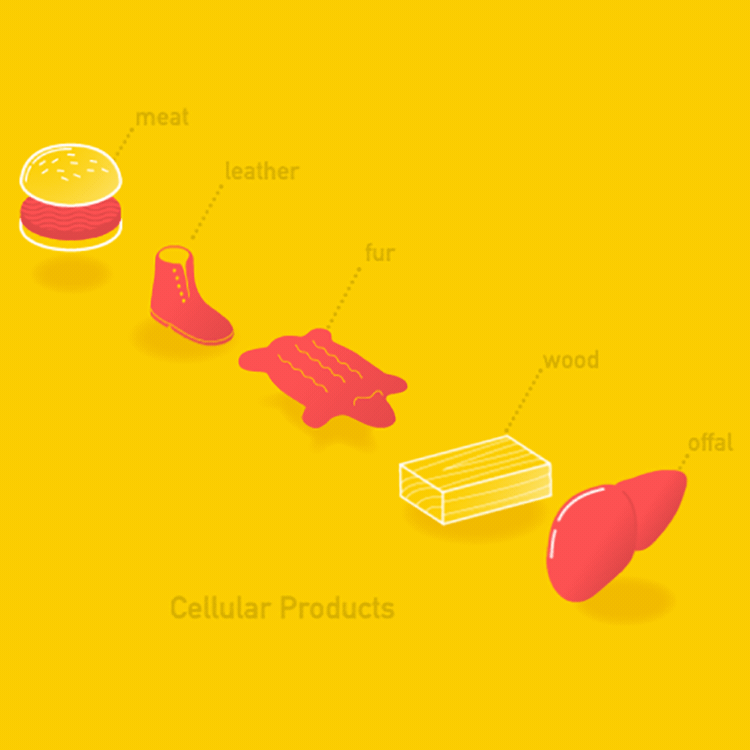
Agricultural products can be classified as acellular (without cells) or cellular (containing cells).
How to Make Acellular Products with Cellular Agriculture
Acellular animal-sourced foods (like milk) can be made without animals by using a microbe like yeast or bacteria.
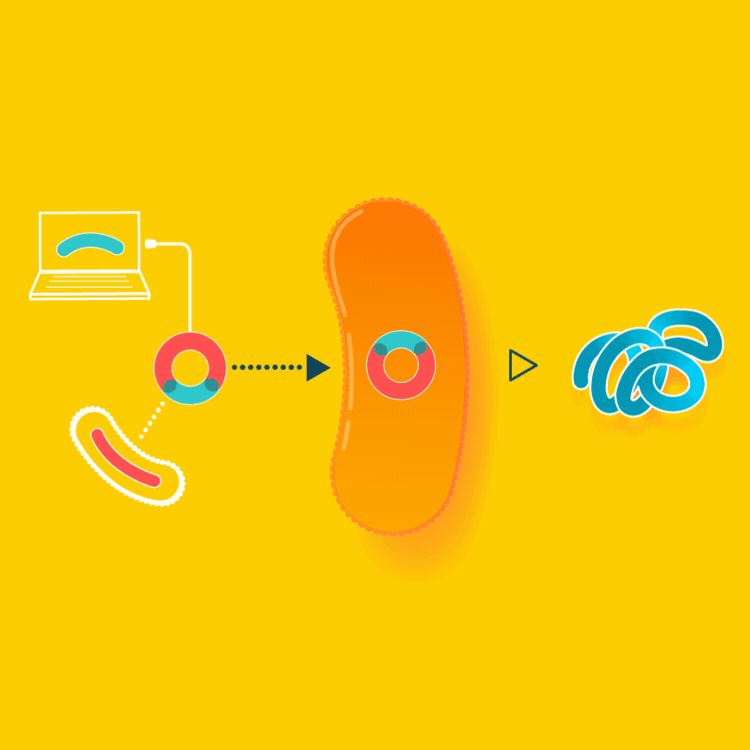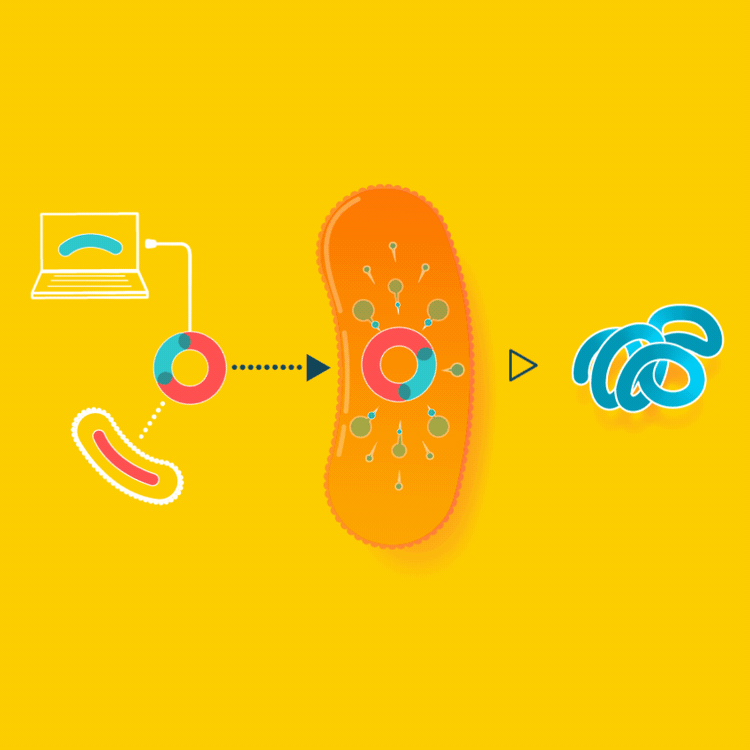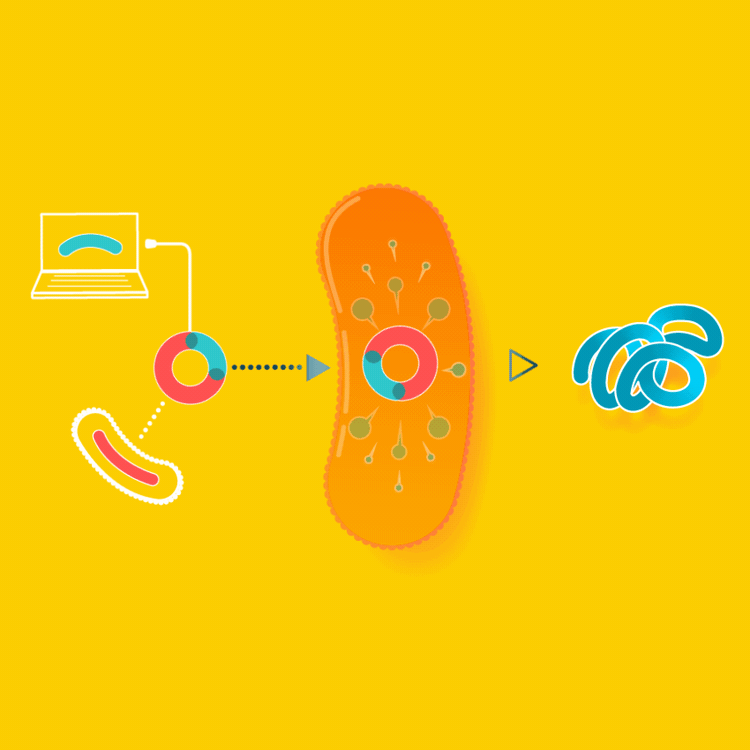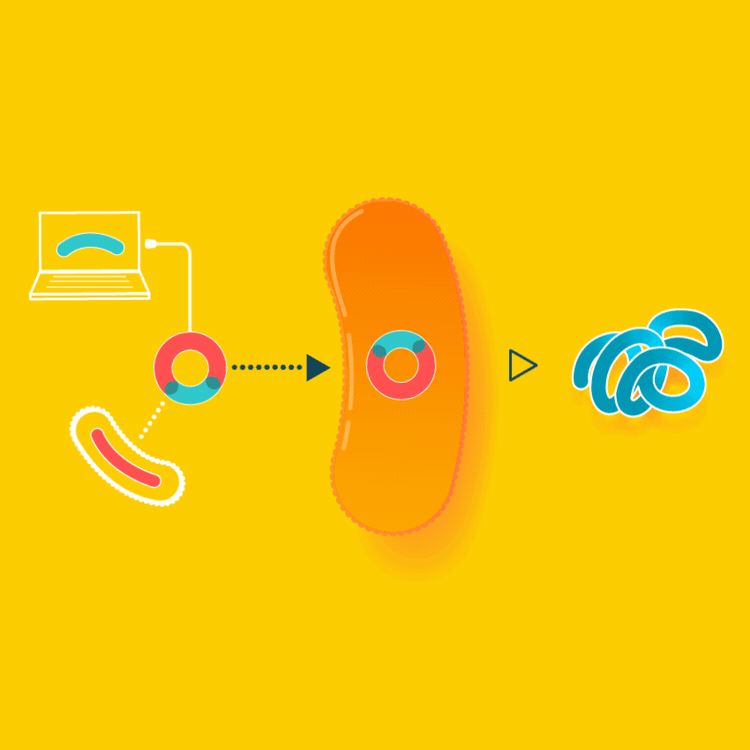
To create a starter culture that can make animal proteins, look up the gene for the protein online and insert the protein gene into a microbe. The microbe will now be able to make the protein you were looking for. You will only have to create this starter culture once.
In the example of milk made with yeast, the yeast are altered by inserting into them the gene carrying the blueprints for casein, a milk protein. Since all cells read the same genetic code, the yeast, now carrying so-called recombinant DNA, makes casein identical to the casein cows make in their milk.
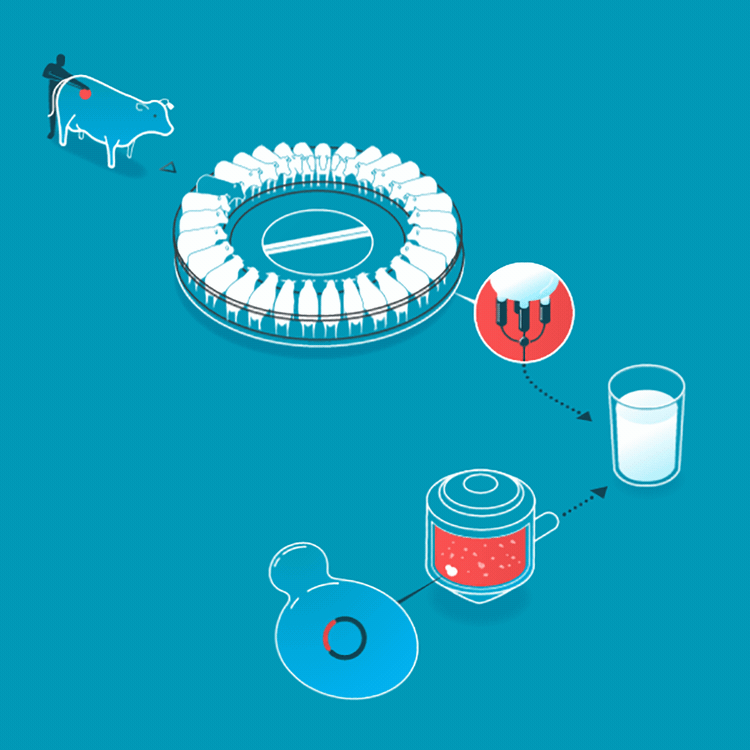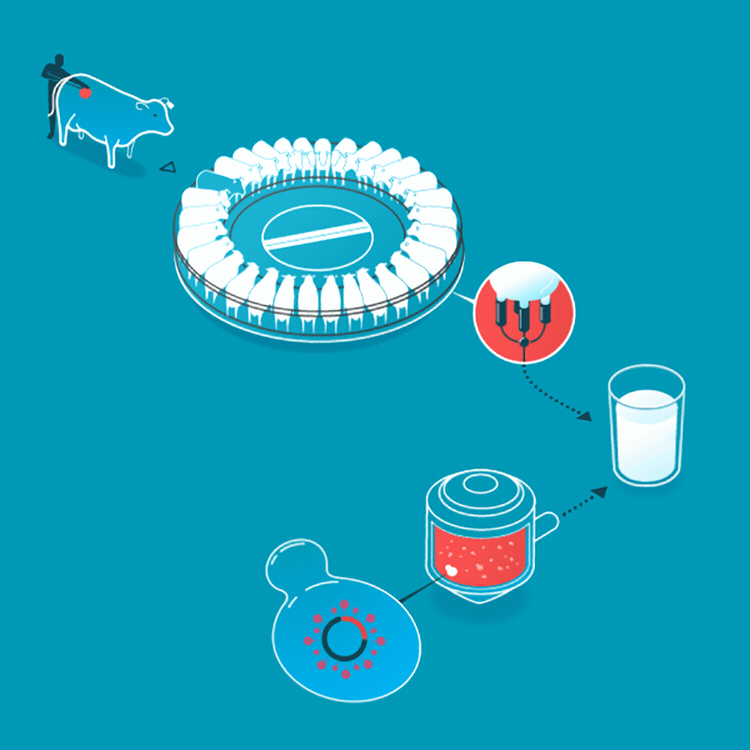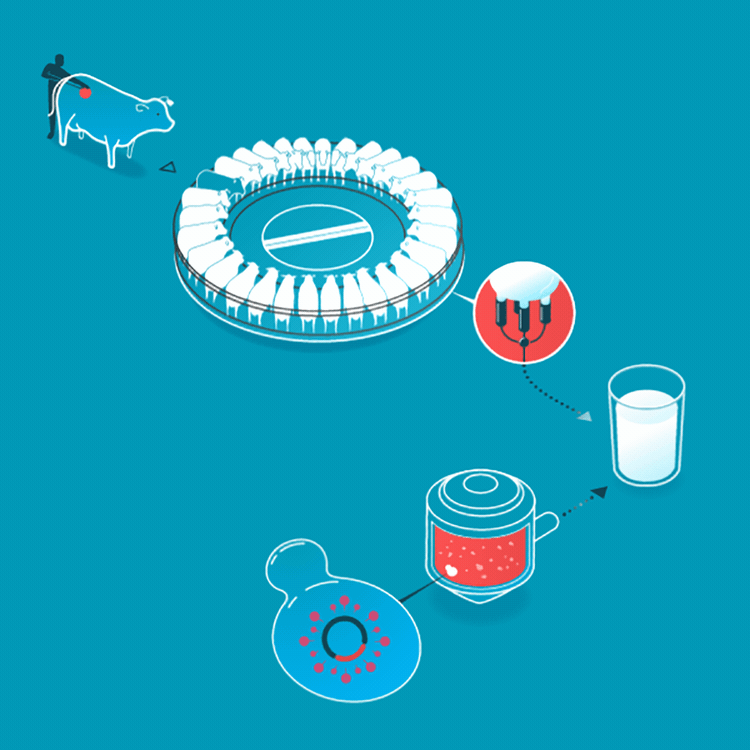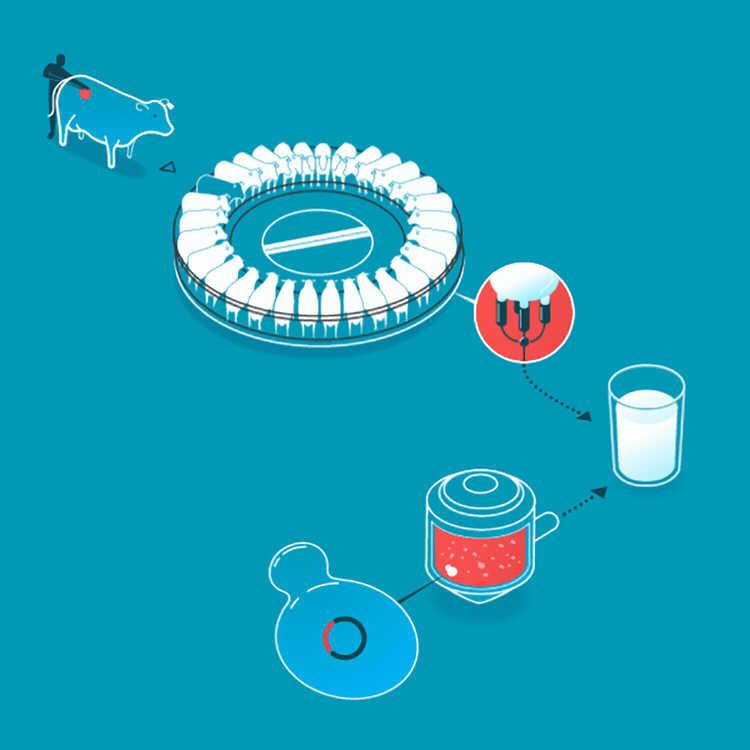
Milk is usually made by mother cows kept in a lactating state in an industrial setting. Instead, we can make the exact same milk by brewing it, using a culture that consumes simple sugars to make milk proteins.
We have made acellular animal products in cell cultures before.
Animal insulin could be considered the first cellular agriculture product. In 1922, Frederick Banting, Charles Best, and James Collip treated the first diabetic patient with an insulin injection. Thereafter, insulin was collected from the ground-up pancreases of pigs or cattle. But in 1978, Arthur Riggs, Keiichi Itakura, and Herbert Boyer were able to insert the gene carrying the DNA for human insulin into a bacteria, so the bacteria could make insulin identical to the insulin that humans make. Today, the vast majority of insulin is made by this engineered yeast or bacteria. This has made the insulin supply safer, more consistent, and identical to the insulin humans produce.
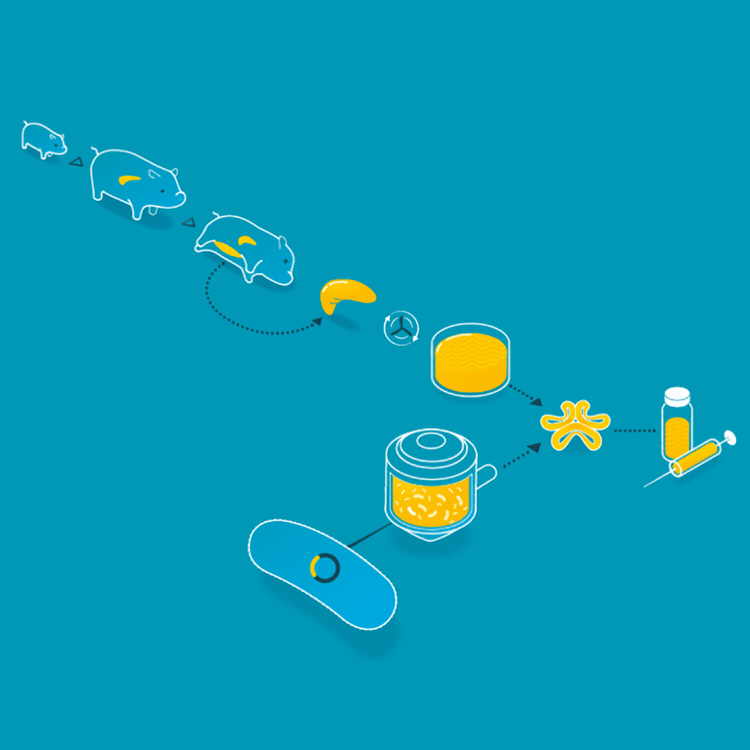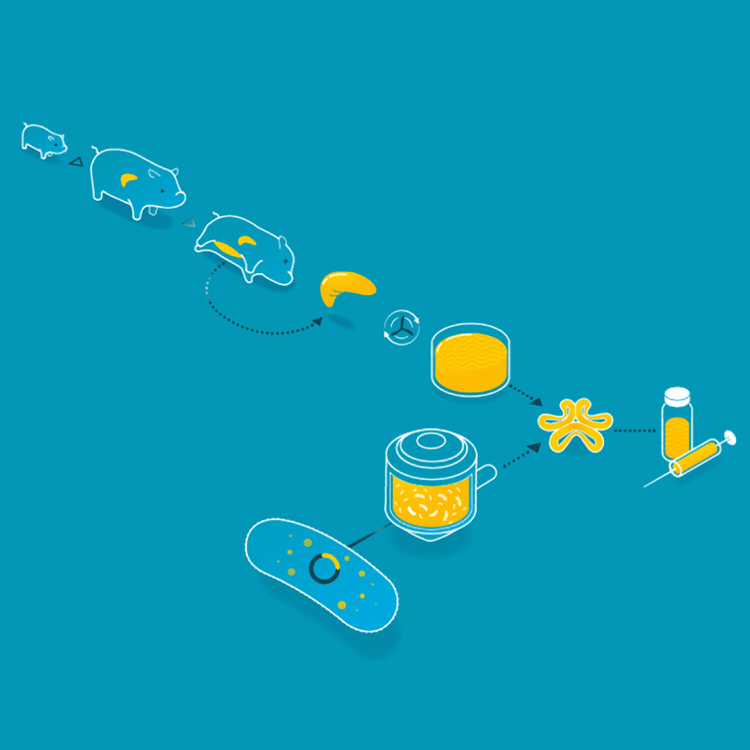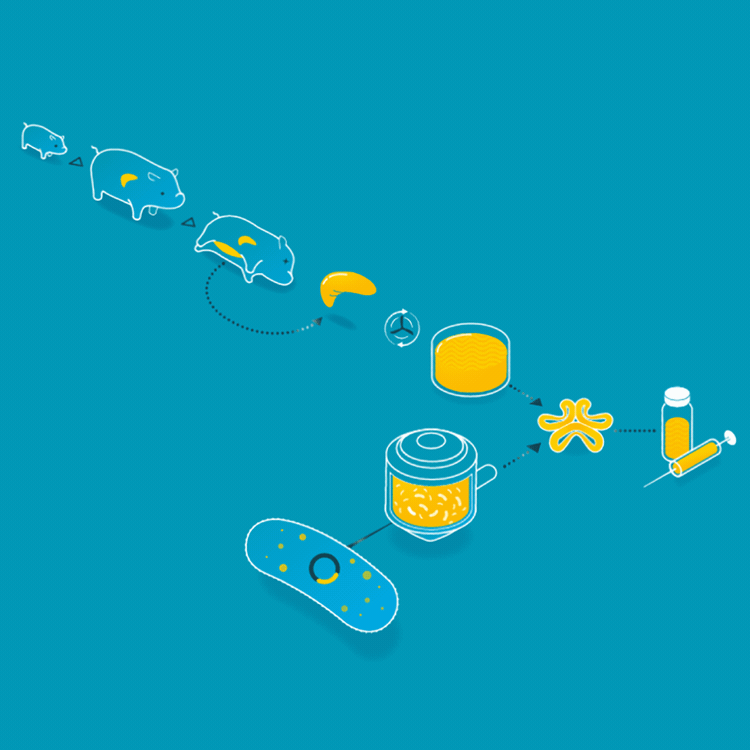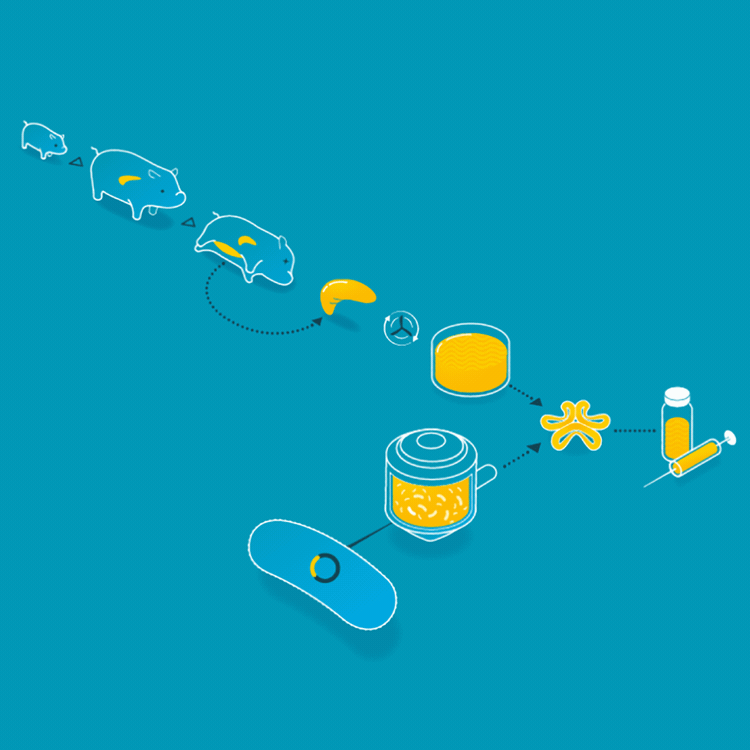
For the first 60 years of its use as a treatment for patients with diabetes, animal insulin was collected from the ground up pancreases of pigs and cattle. Today, it is made by microbes who produce the human form of insulin.
We make a food animal product without animals already, too. Rennet is a mixture of enzymes that turns milk into curds and whey when making cheese. Traditionally, rennet is extracted from the inner lining of the fourth stomach of calves. On March 24, 1990, the FDA approved a bacteria that had been genetically engineered to produce rennet, making it the first genetically engineered product for food. Today, the majority of cheesemaking uses rennet enzymes from genetically engineered bacteria, fungi, or yeasts. Rennet harvested from cell cultures is purer, more consistent, and less expensive than animal-derived rennet.
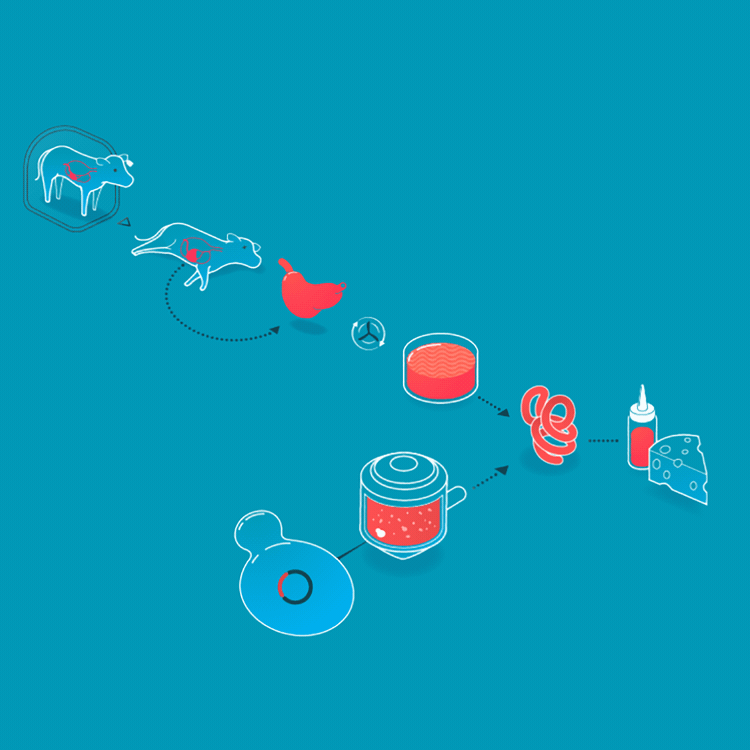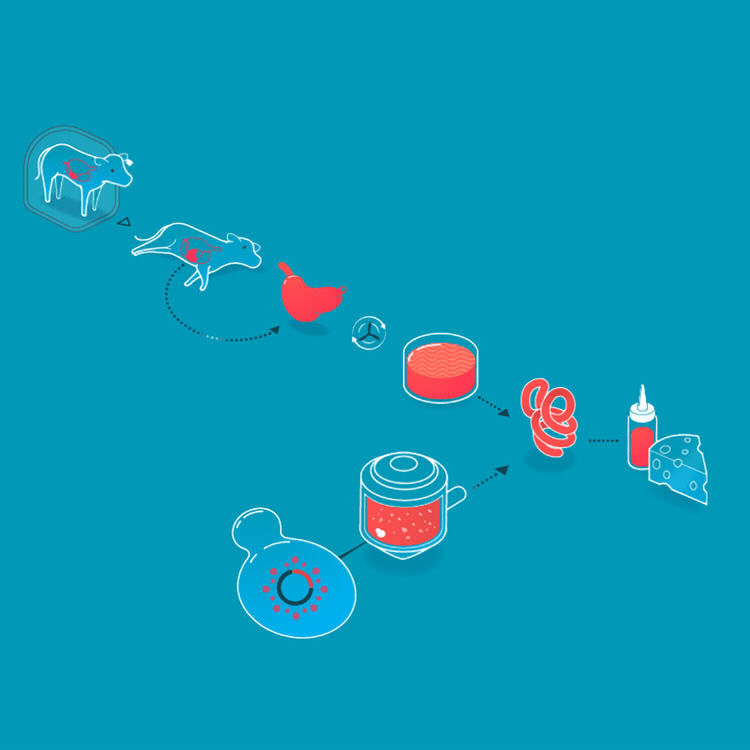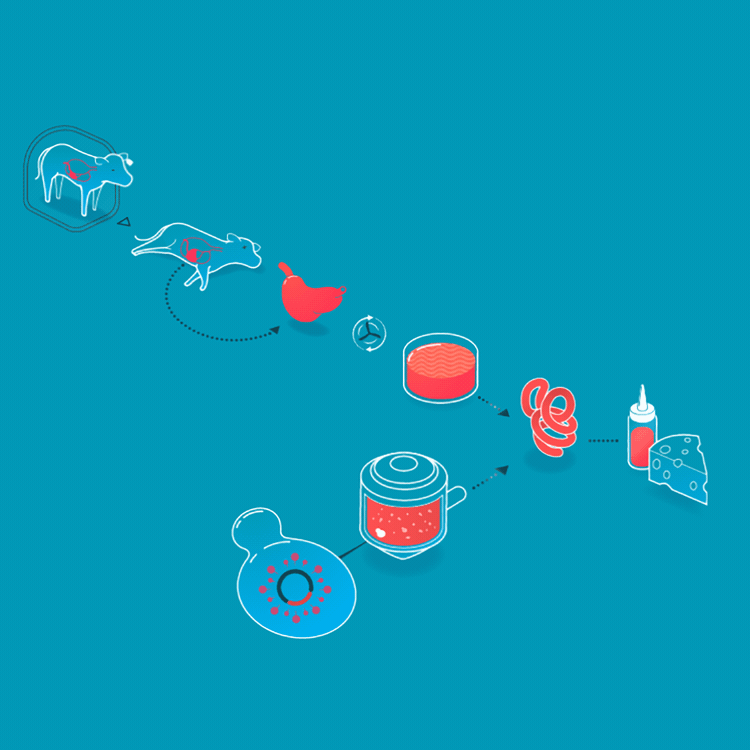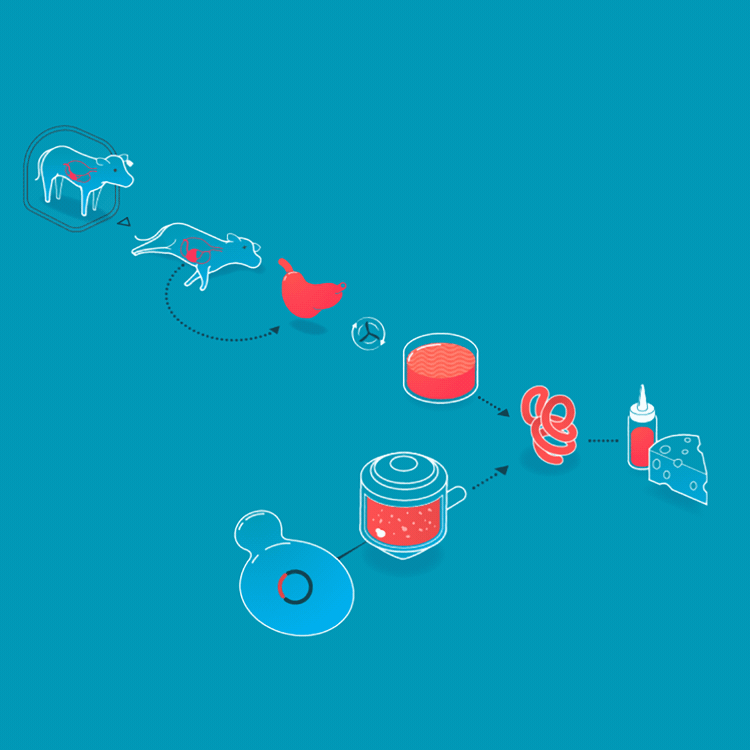
Rennet originally was collected from the fourth stomach of young calves. Today, it is made by microbes who produce rennet enzymes.
These examples go beyond animal-sourced compounds and also apply to plant compounds too. All of this is possible because, as said above, all cells use the same genetic code.
A Swiss company called Evolva is making vanillin (the primary component of vanilla flavor) from yeast. The vast majority of vanillin is produced from petrochemicals or chemically derived from lignin (a constituent of most plant cell walls). The small percentage of vanillin from vanilla beans is harvested in tropical forests from the vanilla orchid. A cultured vanillin would avoid rainforest farming and chemical synthesis of vanilla.
Ginkgo Bioworks, a company based in Boston, is using cellular agriculture to produce flower fragrances from engineered microbes rather than from flowers.
How to Make Cellular Products with Cellular Agriculture
Most cellular products exist in tissues. In cellular agriculture, tissues are made outside the body in a process called tissue engineering. Cells from a particular species and tissue type are assembled on a scaffold (to grow on) with serum (food for the cells to feed on while they grow) in an environment that promotes growth.
Today, tissue engineering is a relatively new scientific pursuit, with a focus on clinical applications such as growing skin for burn victims or organs for patients requiring organ transplantation. The focus is on the tissue having a biological function — in other words, the tissue-engineered organ needs to be able to work in a living person.
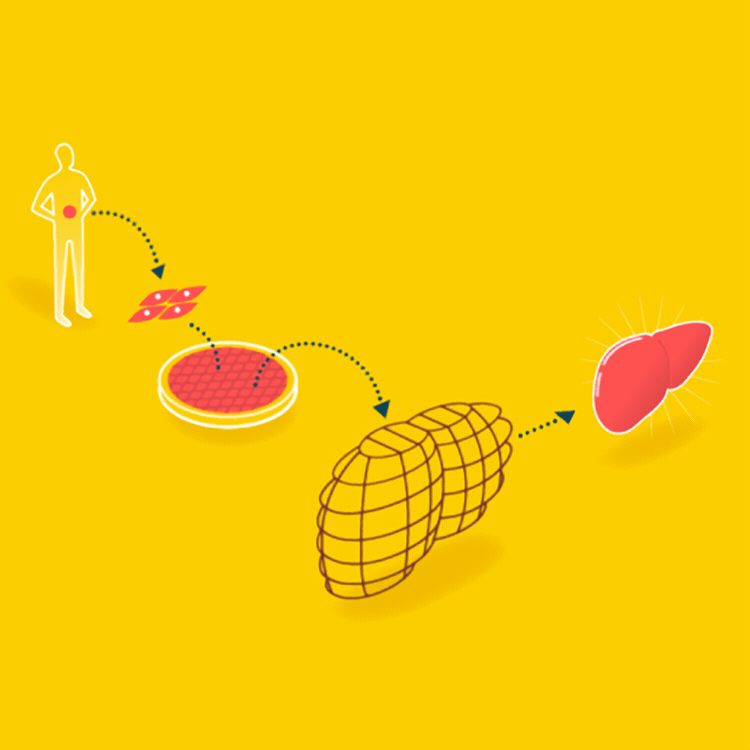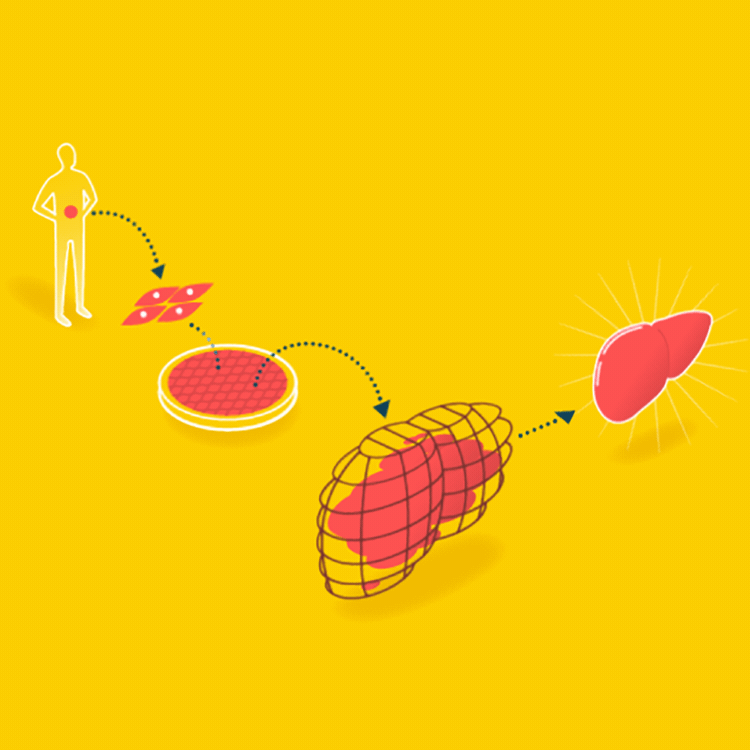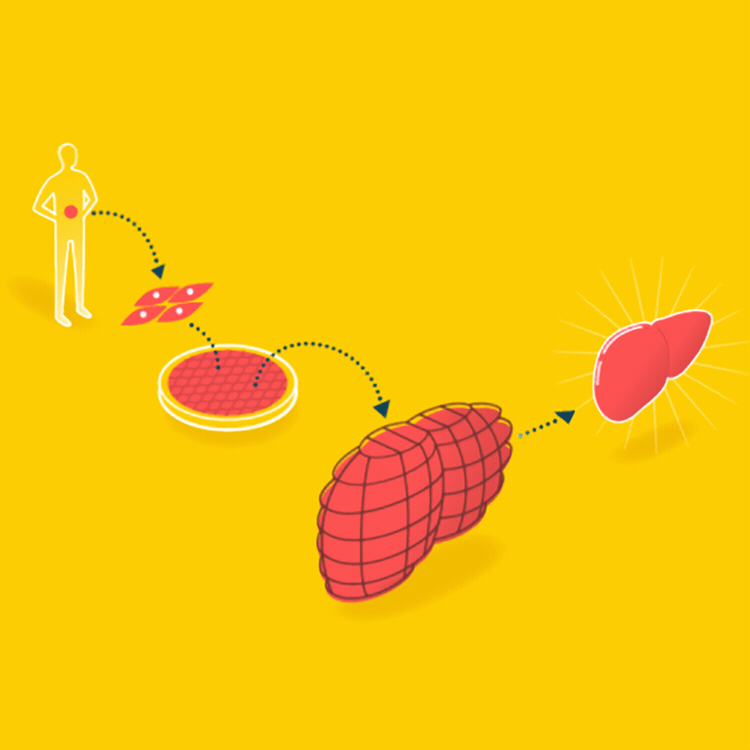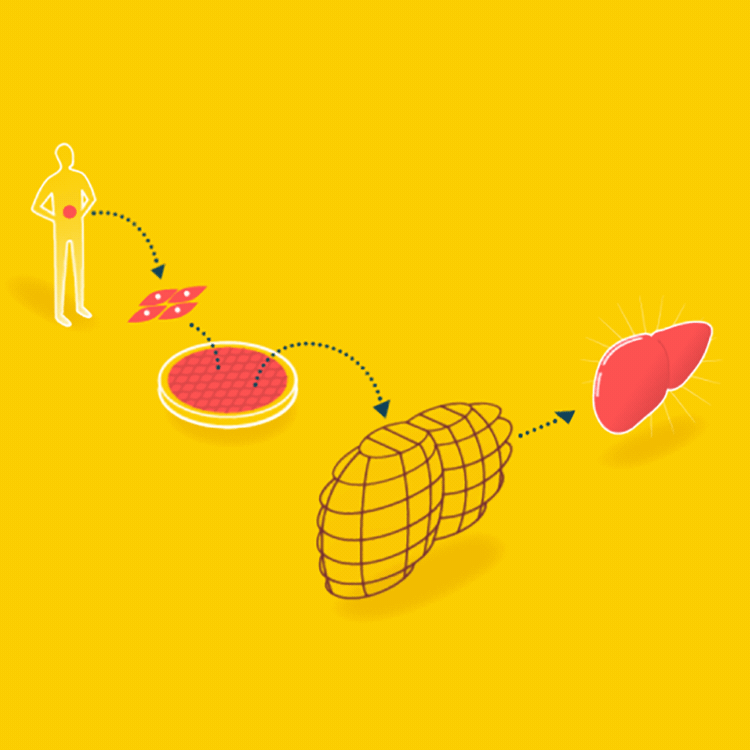
A major goal in tissue engineering today is to grow functional organs for patients. A biopsy is taken from the patient, and the organ is grown with the patient’s cells on a suitable scaffold. The goal is an organ that can be transplanted into the patient without being rejected.
While the science behind growing tissue for an organ transplant is similar to growing muscle tissue for food, both come with a set of very different considerations. For example, tissues for meat or leather do not need to work as an organ in someone’s body. Instead, meat needs to have a particular nutritional value, mouthfeel, or taste. Leather needs to have a certain strength, texture, or softness. All cellular agriculture products need to be made affordably — that means producing tissues at a scale much larger than what is required for patients requiring organ transplants.
In our bodies, blood vessels bring nutrients and remove waste products from our tissues. This allows the tissues in our bodies to be quite thick. But if you do not have vessels, the cells do not have access to what they need to grow. In culture, tissues can only grow about 0.2 mm thick without vessels. For growing organs for medical purposes, this is a problem. But for growing cultured meat, it may not be.
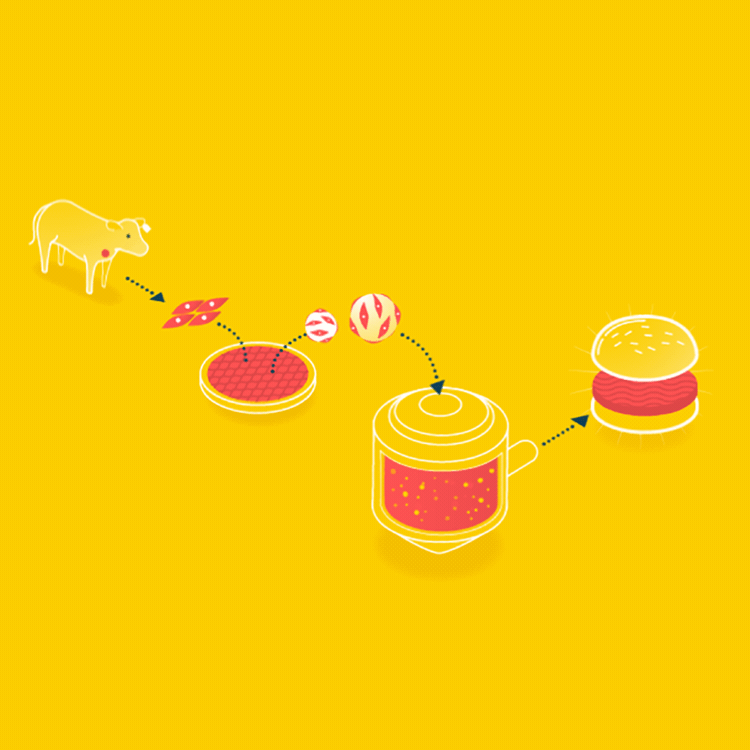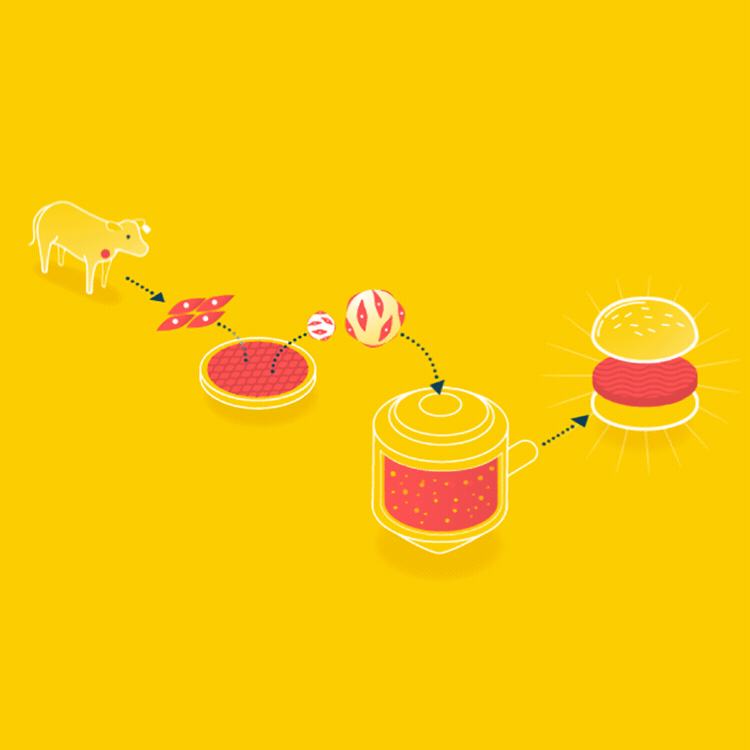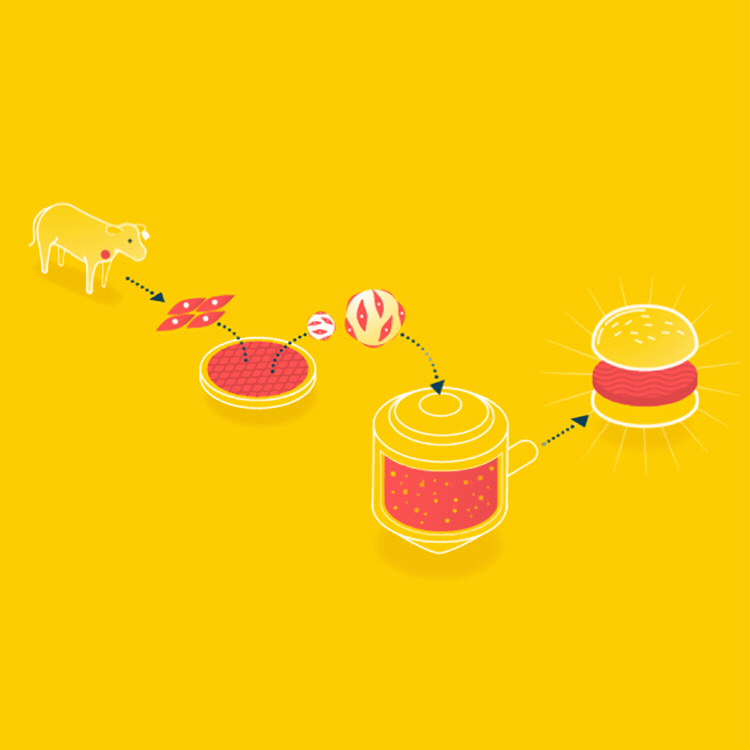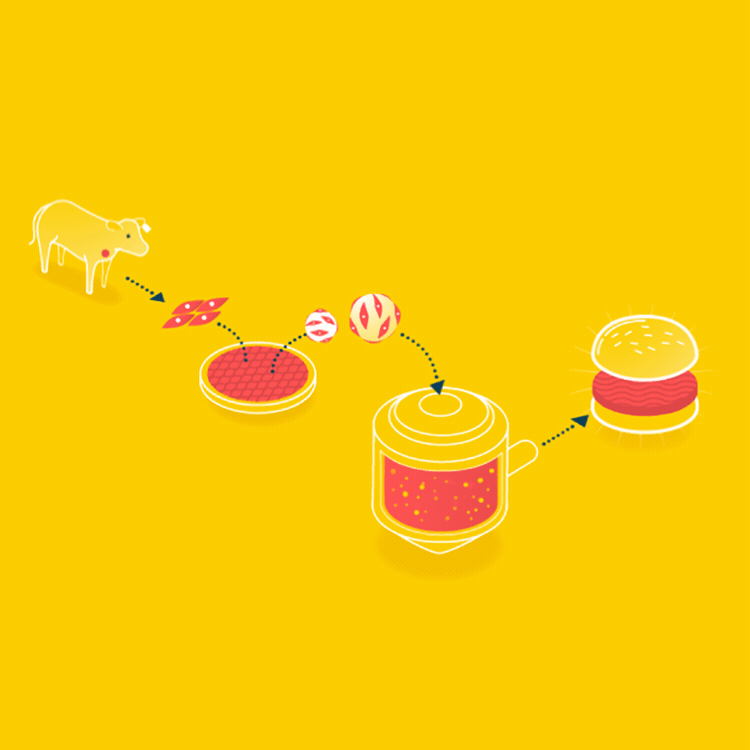
Because cells can only grow about 0.5mm thick in culture, it is easier to grow ground meat than something thick like a steak. Muscle cells could be grown on beads, which offer a lot of surface area, in a bioreactor, and when the muscle cells are removed, it will already have the consistency of hamburger.
The Benefits of Cellular Agriculture
Compared to their conventional counterparts, cellular agriculture products have fewer environmental impacts, a safer, purer product, and a more consistent supply. This is because the product is being produced in safe, sterile, controlled conditions.
Another exciting aspect of cellular agriculture is the ability to design and tune what you are making. For instance, you could make meat with fewer saturated fats and more unsaturated fats, or you could make leather of different thicknesses. You could make milk without lactose, or eggs without cholesterol.
Despite the benefits and opportunities presented by cellular agriculture, it remains an underfunded area of research.
Have more questions about cellular agriculture? Ask us!

